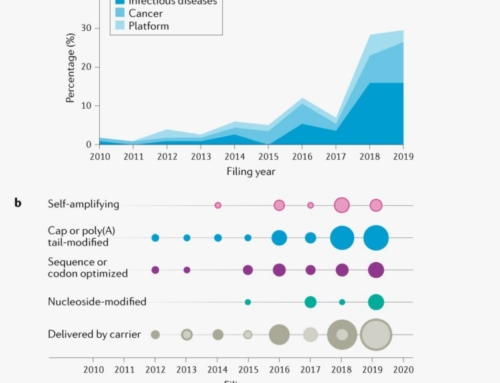
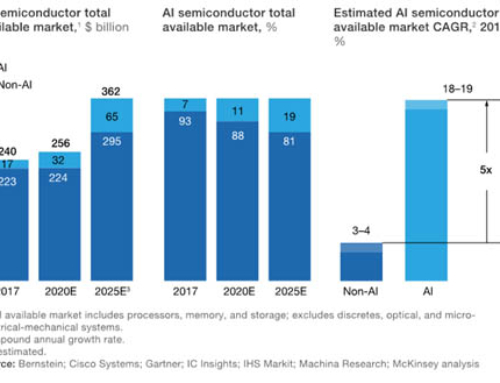
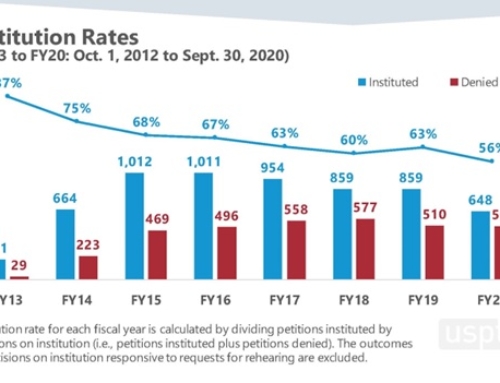
By Chie Mueller-Hillebrand Ph.D., David Adamovich Ph.D., and Drew Lowery, Ph.D.
December 10, 2020
Japan is not the first country that comes to mind when Senior Managers and IP Attorneys within the Massachusetts Biotech sector discuss leading edge biotech research and its implications for research directions and IP. However, as the Nobel Prize awards to Tasuku Honjo (2018) and Shinya Yamanaka (2012) of Kyoto University clearly demonstrate, many breakthroughs in cancer therapies originated there. Much of these scientists early work, including research and patent filings, was published only in Japan, in the Japanese language, and not in English. As a result, U.S. companies that failed to monitor Japanese language patents and literature were blindsided by this work. In this article we highlight several fields where Japan is a leader in biotech research, and illustrate how accessing Japanese language documents facilities more effective decision making in such areas as R&D, licensing, and IP strategy.
Tasuku Honjo’s group discovered the function of Programmed Cell Death 1 (PD-1) in 1992, which subsequently led to the discovery of a whole new class of immune checkpoint molecules that regulate T cell activation. Some cancer cells are able to over-express PD-1 on their surface and evade the host immune response, leading to unchecked cell expansion. Since he was the first to discover PD-1, Honjo had a head start on everyone else for both developing and commercializing anti-PD-1 antibody therapy for treating cancer and filed early patent application to protect future market share. By 2005, a collaboration with Ono Pharmaceuticals and Medarex led to the development nivolumab which was approved for use in humans as a first in class cancer therapy (Opdivo) in 2014. By 2018, Opdivo became the top immune oncology drug in the world, with over $4.2 billion in sales in the US alone.
In 2006, Shinya Yamanaka made a discovery that revolutionized stem cell research and therapies. He found that a mixture of just 4 transcription factors — Oct3/4, Sox2, Klf4, and c-Myc — were sufficient to reprogram somatic cells into induced pluripotent stem cells (iPSCs). This discovery now meant that any research organization or therapeutics company would have the ability to generate their own stem cells for research rather than acquire cells sourced from embryonic or fetal tissues. With the knowledge of this discovery, a venture company originating at the University of Tokyo and Kyoto University founded ReproCELL, the first company to produce commercially available iPSCs.
Access the full article
By submitting your information to access this article, you consent to Global Prior Art to use this information to keep you informed about products, services and events.
This paper was originally published in the BPLA Q4 Newsletter